

What are elements? There are many different types of atoms, called elements. Hydrogen, carbon, oxygen, sodium, and chlorine are a few different elements. The number of protons an atom has determines which element it is.
The simplest type of atom, which only has one proton, is the element hydrogen. Unlike every other element, hydrogen doesn't have any neutrons. The element oxygen is more complex; oxygen atoms have 8 protons. Your body is made of a whole lot of atoms of these two elements (hydrogen and oxygen)!
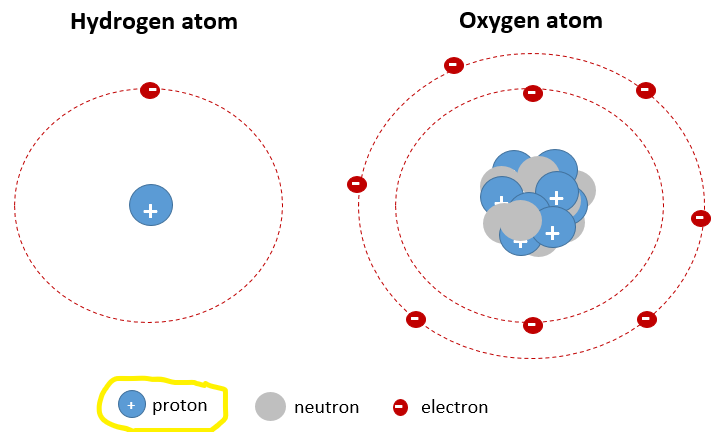
Atoms in their basic "elemental" forms have no "net" charge. That is, they have the same number of (negatively charged) electrons (e-) and (positively charged) protons (p+). So, hydrogen atoms have one electron. Oxygen atoms have 8 electrons.
The elements we have discovered (so far!) are shown in Periodic Tables like this one. Elements in Periodic tables are ordered by "atomic number," which is the number of protons the element has. The atomic number/number of protons is shown in the top right corner of each box for each element. For example, as you can see in the Periodic Table, the element Sodium (Na) has 11 protons.
Ions of elements: Sometimes elements gain or lose one or more electrons. Because the atoms no longer have the same number of protons (+) and electrons (-), they have an electric charge. Atoms that have lost or gained electrons are called ions.
You've reached the end of this unit. You can click on the "Chemical & Physical Reactions" button to see more units related to your research question.