

Coulomb's Law. The strength of the electric force between two charged particles is related to two variables: (a) the distance between them (r), and (b) the "net" charges of the particles. We'll talk about how the strength of the electric force (Fe) changes with distance first.
Distance. There is a strong relationship between the strength of the electric force, Fe, and distance between the charged particles. The relationship between the strength of the attractive or repulsive force between charged particles (Fe) and distance between the particles (r) is shown below:
The equation above shows that if the distance between the charged particles is very very large (let's say one million meters), the force between them would be very small. But when the particles are very close to each other, the force between them gets pretty big.
The "net" charge of the particles. As you might expect, the strength of the attractive or repulsive force between the charged particles is also proportional to the net charge of each of the particles (represented as "q").
The net charge of a particle is the difference between the number of electrons and protons the particle has (since positive and negative charges "cancel each other out"):
So, if we have two charged particles, the electric force is proportional to the net charge of each particle:
Fe ∝ q(net)1
Fe ∝ q(net)2
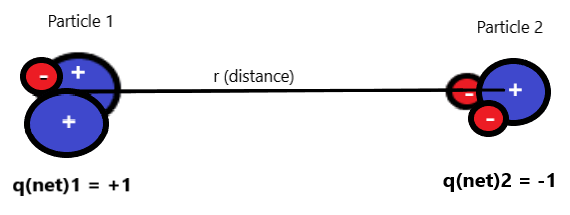
In the figure above, Particle 1 is made of 2 protons and one electron (for a net charge of +1). Particle 2 is made of 2 electrons and one proton (for a net charge of -1). The net charges of +1 and -1 are +/-1 times the charge of one proton/electron.
We can combine the relationships between Fe and r and Fe and q(net) into one equation, called Coulomb's Law...
Coulomb's Law. We know that the strength of the electric force between charged particles increases as (1) the particles get closer to each other and (2) as the total charges of the two particles increases. The exact strength of the electric force between two charged particles can be calculated by using Coulomb's Law (below):
Fe = ke
×
q(net)1*q(net)2
r2
(Note: The charges of protons and electrons are given in the unit called "Coulombs." One proton is equal to +1.6 × 10-19 Coulombs. An electron has the same sized charge as a proton, but is negatively charged (-1.6 × 10-19 Coulombs). To simplify in the example below, we'll call the charges of one proton or electron "one charge unit.")
Applied example: Say you rubbed an initially uncharged balloon on your hair, which was also initially uncharged. This transferred 50 electrons from your hair to the balloon. Your look-alike twin rubs another (initially uncharged) balloon on their hair, which transferred 100 electrons from the balloon to their hair. Your twin's hair would be pulled by the balloon with four times the force your hair would be pulled with.