

What are Crystals? When you think of crystals, you may think of pretty gems that are used in jewelry, like diamonds (April birthstone), amethysts (February birthstone), and peridot (August birthstone). Some different types of crystals are shown below. Crystals like the ones shown here are defined as solid substances whose molecules are arranged in a repeating pattern.

Other examples of crystals include table salt (NaCl), sugar (C12H22O11), and ice (H2O in its solid form). With the exception of crystal water (ice), all the examples of crystals here (salt, sugar, diamonds, amethysts, peridot, and all the crystals shown above) are solids at room temperature.
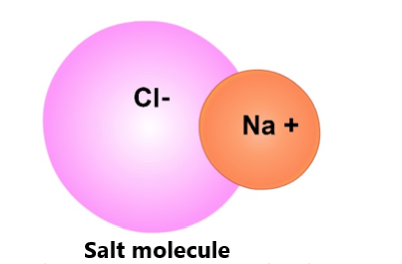
Table salt is one type of crystal. Crystals are defined as solid substances whose molecules are arranged in a repeating pattern. It is this repeated molecule arrangement that gives crystals qualities like shininess and colors that makes them look pretty to us.
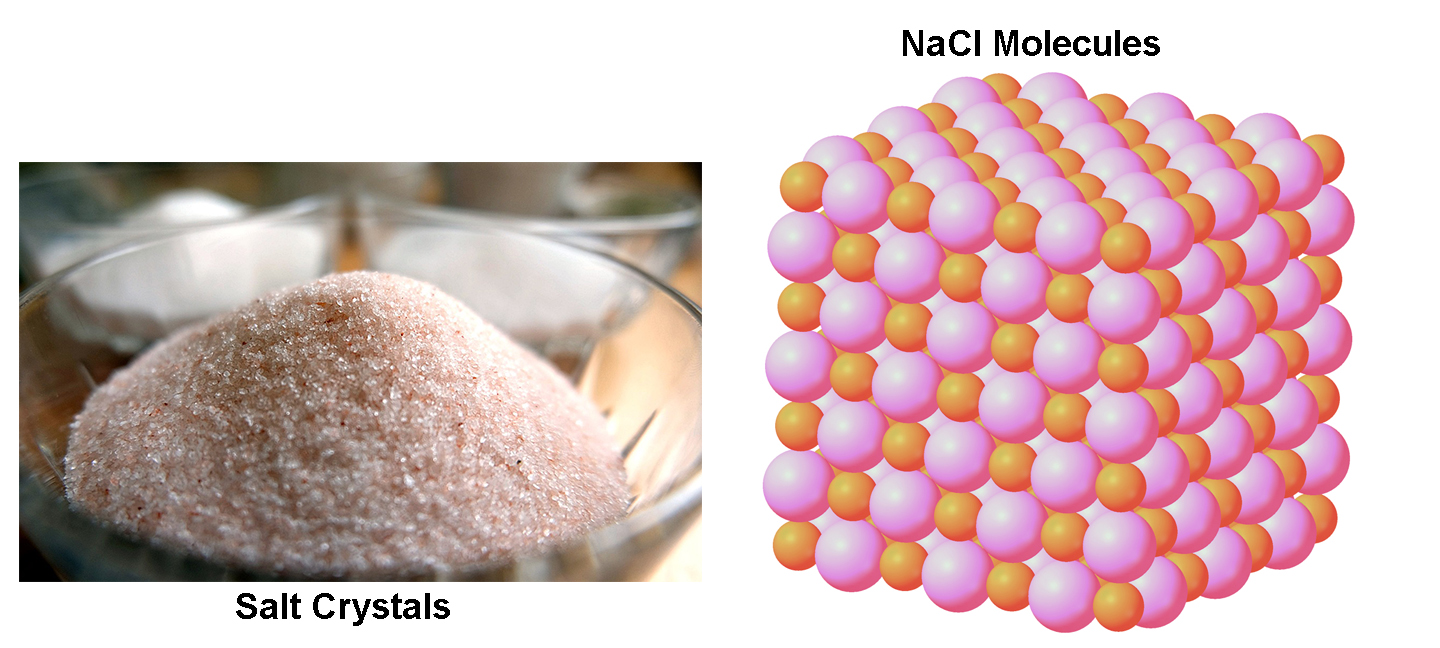
Ice (the solid form of water, or H2O) is another example of a crystal. This means that
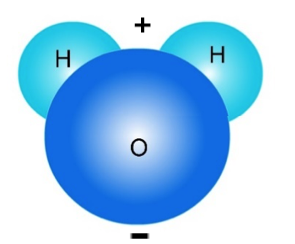
A water molecule is shown below.
In ice, H2O molecules are arranged in repeating patterns. There are many types of patterns that H2O molecules in ice can form. Two possible patterns are shown below. The pictures below are "ball and stick" representations of ice crystals: regular patterns of H2O molecules. Oxygen atoms are shown as darker blue balls, hydrogen atoms are shown as lighter blue balls. The bonds between atoms are black lines (solid to the left and dashed to the right). (Remember that bonds are not physical "things", like atoms, and just represent attractive forces between certain atoms.)
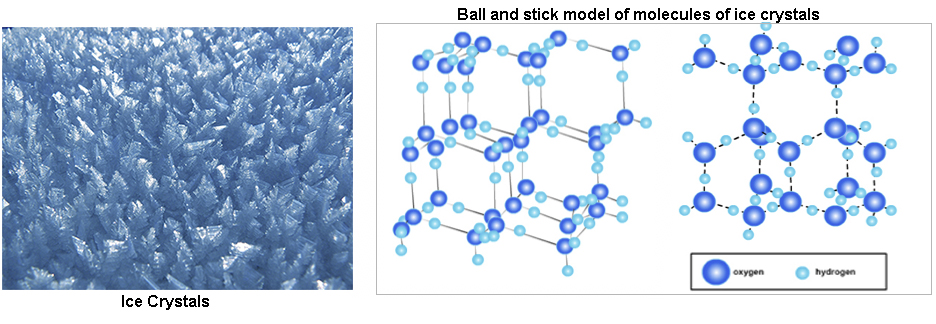
In both patterns, notice that the hydrogen atoms (smaller) in general are closer to oxygen atoms (larger) than other hydrogen atoms. And the oxygen atoms are closer to hydrogen than other oxygen atoms. (Some oxygen atoms look like they are closest to other oxygen atoms, but that's because it's hard to show 3 dimensions in a 2 dimensional picture, and the hydrogen atoms may be hidden by oxygen atoms that are in front of them.)
 Common Misconceptions about Crystals
Common Misconceptions about Crystals