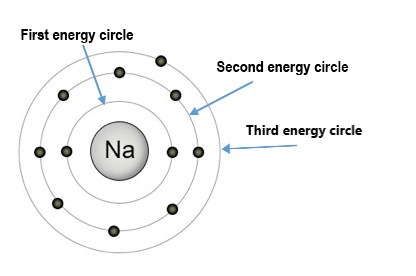
An interesting fact about atoms is that their electrons can only have certain amounts of energy. The electrons can't just have any amount of energy. The energies that electrons are "allowed" to have are represented by Energy circles.
The energy levels of electrons within atoms represent the total energy of the electrons, and include both their Kinetic and Potential energies. Interestingly, the kinetic energy of electrons actually decreases as the energy levels get higher (or are farther from the nucleus of the atoms). However, the potential energy of the electrons due to the attractive electric force between the electrons and protons in the nucleus increases as electrons go to higher energy levels (farther from the nucleus). The total energy increases in orbits that are farther from the nucleus because the increase in potential energy is greater than the decrease in kinetic energy with increasing energy levels. As a result, there is a net gain in energy.
In other words, electrons have the least amount of total (kinetic & potential) energy at the first energy circle. They have the second-least amount of total energy at the second energy level. Etc.
Below is an example of a sodium (Na) atom with its three Energy levels (or Energy Circles) shown: