

What are Molecules? Atoms can be attracted to other atoms. When this happens, they form groups of atoms called molecules. Molecules are two or more atoms that are bonded together through some attractive force (often, the electric force).
Molecules can be made of atoms of the same type. For example, an oxygen molecule, O2, is made of 2 oxygen atoms.
Or, molecules can be made of atoms of different types (or different elements). For example, a water molecule, H2O, is made of two different elements: hydrogen (H) and oxygen (O).
As you've probably noticed, the name of a molecule (such as CO2) is abbreviated by combining the symbols for elements that make up the molecule. For example, the molecule "water" is written as "H2O," which combines the symbols H (for hydrogen) and O (for oxygen). You can tell how many different elements are in a molecule by the number of capitalized letters there are (since element symbols are one or two letters beginning with a capital letter). Since there are two capitalized letters in "H2O," we know there are two elements (H and O).
The number after each element shows the number of atoms of that element in the molecule. If there is no number after the element, that means there is just one atom of that element. For example, in H2O, the "2" after H means that there are 2 hydrogen atoms in a molecule of H2O. There are no numbers after "O," which means there is just one oxygen atom.
Example 1: O2. Two oxygen atoms may bond together to form an O2 molecule (the "2" after the "O" means that there are two Oxygen atoms in this molecule). Below are two different ways of representing bonded Oxygen atoms that form the molecule O2. The picture on the left shows a very simple "ball" model of an oxygen molecule:
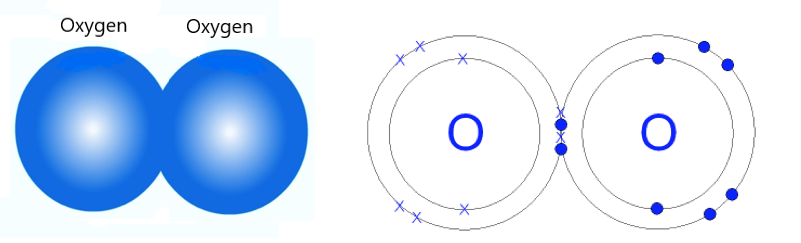
The picture above on the right shows a more detailed model. Here, the "O"s (for "Oxygen") are shown in place of the nucleus. This is just a simpler way to represent the nucleus of an oxygen atom. The electrons (e-) from the oxygen atom on the left are shown as Xs and the electrons from the oxygen atom on the right are shown as dots.
The electrons are shown along circles. You may have noticed that there are 4 electrons that are between the oxygen atoms (on the outside circles). The oxygen atoms "share" these 4 electrons.
(Note: The circle that each electron is associated with is not a physical "thing", but represents the amount of energy the electrons on that Energy circle have.)
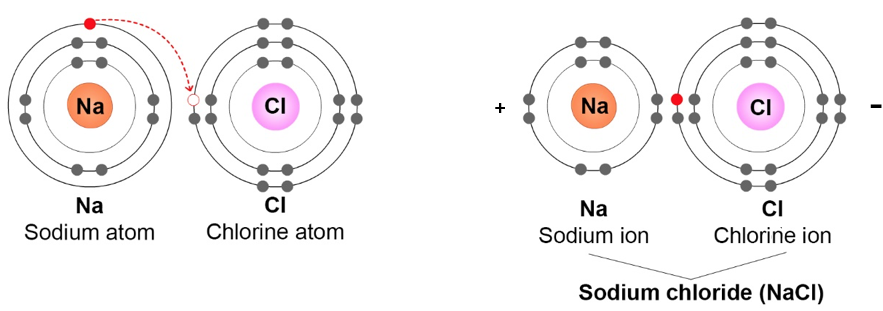
Example 2: NaCl. An atom of sodium (Na) and an atom of chlorine (Cl), bond to make a molecule of NaCl (sodium chloride, or common table salt). Because NaCl molecules are made of two different elements (Na and Cl), NaCl is a compound.
Below are two ways to represent an NaCl molecule.
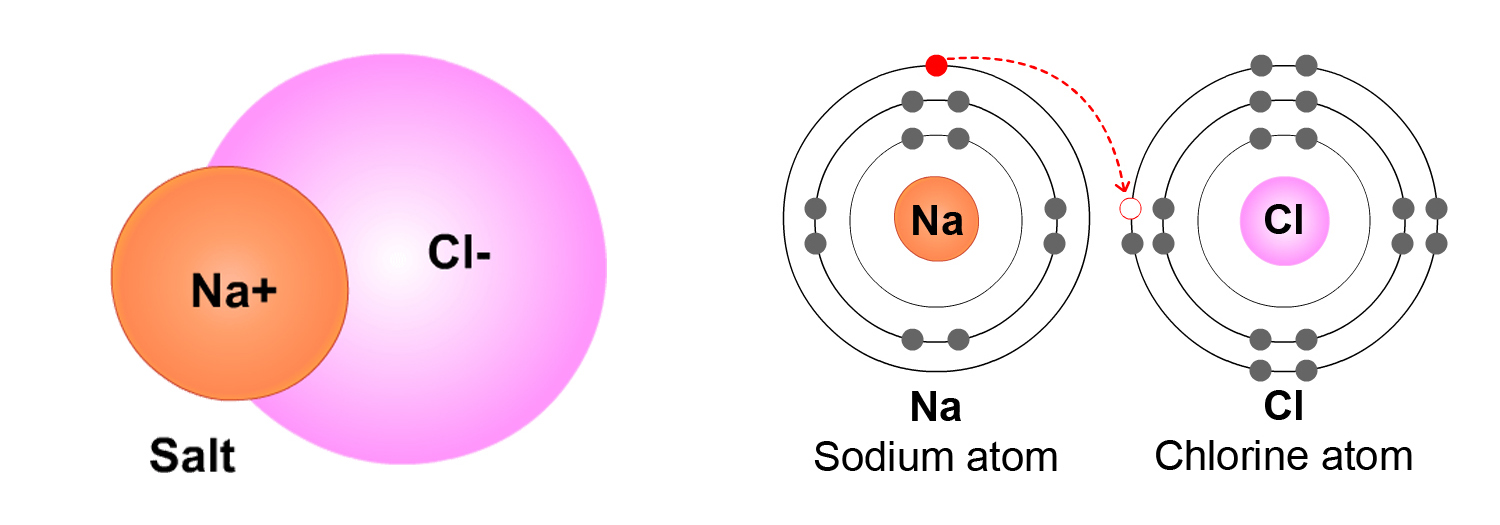
In the picture above on the right, the electrons are shown this time as dots on the Energy circles. This picture shows how an electron (e-) from Na moves to the Cl atom. Because there is an extra electron (e-) on the Cl side of the NaCl molecule, the Cl side is negatively charged. Because the Na atom has lost an electron, it becomes .
Note: In the names of molecules with two elements (like NaCl), the positively-charged atom is written first and the negatively-charged atom is written last. For example, with NaCl, Na is the positively-charged atom and Cl is the negatively-charged atom.
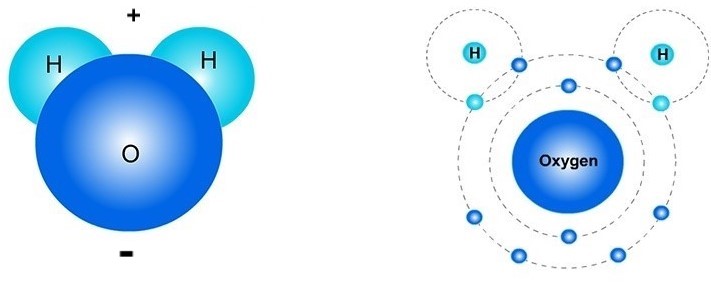
Example 3: H2O. A water molecule (H2O) has two hydrogen (H) atoms (the "2" after the "H" means there are two hydrogen atoms) and one oxygen (O) atom. Some people think a water molecule looks like Mickey Mouse, where its "ears" are the H atoms. Maybe that can help you remember its shape?
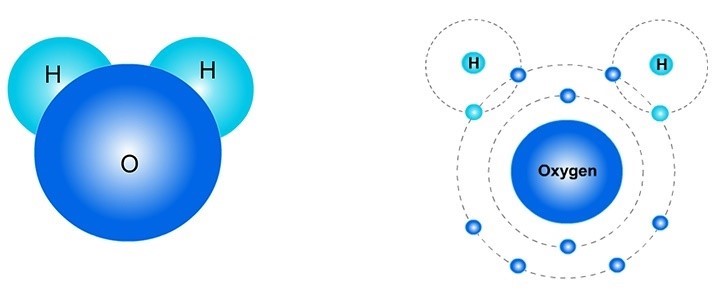
In the picture on the right, there are four electrons (e-) that are shared by the oxygen and two hydrogen atoms. These 4 shared electrons are the two lighter blue electrons (the hydrogen's) and darker blue electrons (the oxygen's) that are in the Energy circle of each H atom. These four electrons are also in the oxygen atom's outer Energy circle. This means that sometimes these electrons "belong" to the hydrogen atoms and sometimes "belong" to the oxygen atom.
Fill-in-the-blank: H2O molecules have .
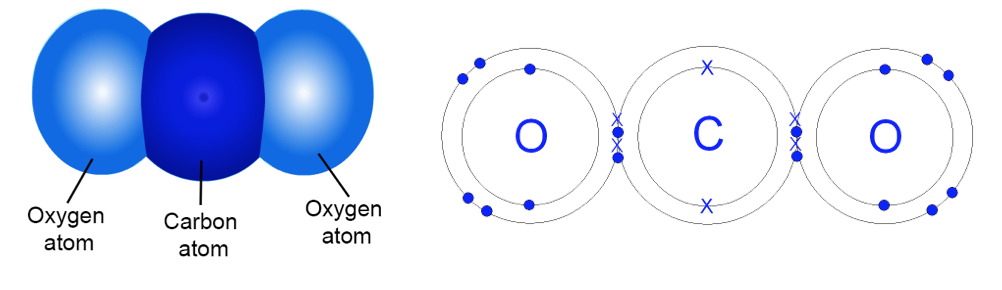
Example 4: CO2. A carbon dioxide molecule (CO2) has three atoms: one carbon (C) atom and two oxygen (O) atoms. Do you see how there are 8 electrons that are between the carbon and each oxygen atom (there are 4 on each side of the carbon's nucleus)? These electrons are shared by the carbon and oxygen atoms.
Example 5: C12H22O11 (sucrose, or common table sugar). A sugar molecule is much more complex! Sucrose, or common table sugar, has 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms.
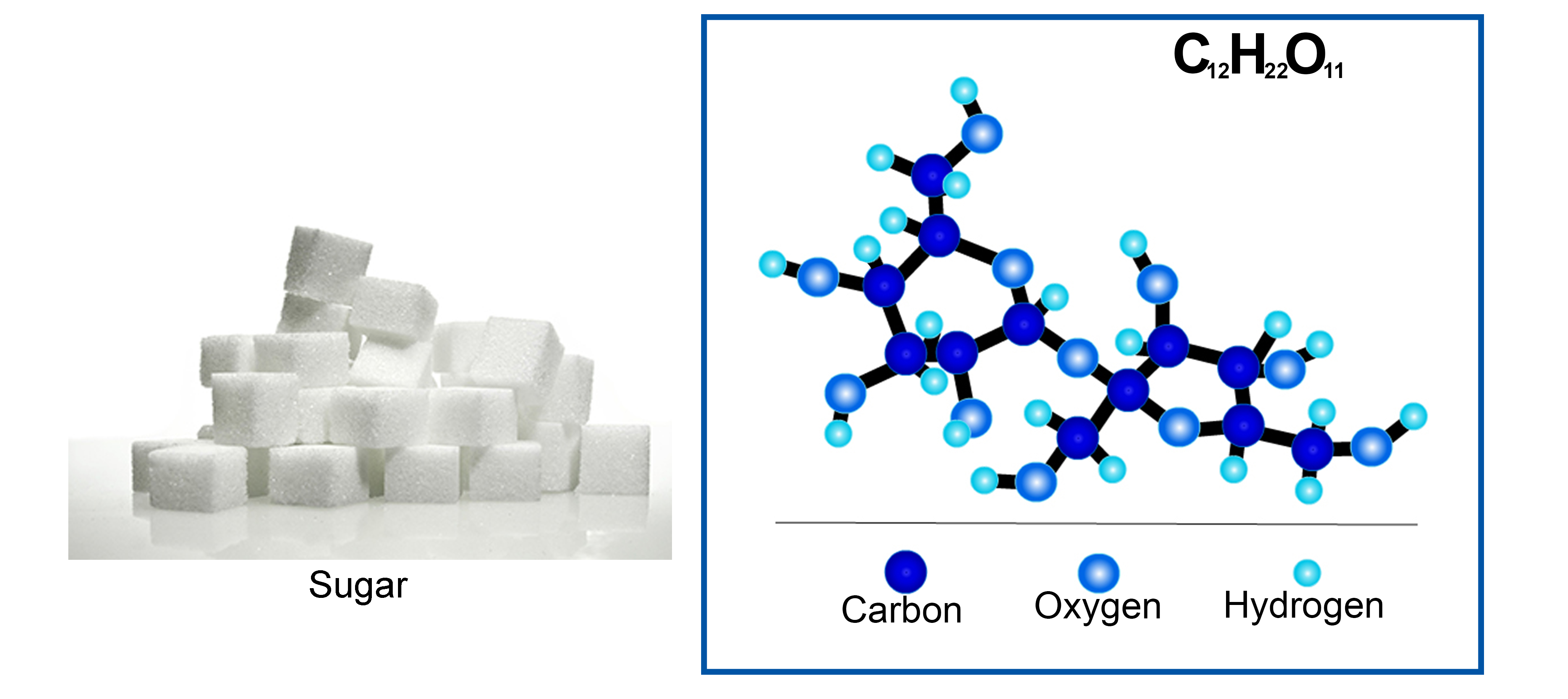
The picture above to the right represents a molecule of sucrose (C12H22O11) or "sugar". This way of representing a molecule is called a "ball and stick" model, where the balls represent the atoms, and the black "sticks" represent bonds between particular atoms.
It is important to understand that bonds between atoms—represented as "sticks" in this picture—are not material or physical objects or things with mass. Bonds are just attractive forces between two atoms (which do have mass), resulting from electric forces between charged particles (electrons and protons).
(A common misconception people have is that "energy" is "contained" or "stored" within bonds. This is false. Energy is an abstract measurement of the motion of objects or radiation.)
Why do atoms bond to form molecules?
It may seem a bit strange, but atoms bond with each other because they "like" it when their outer Energy circle is full. (This is discussed more in the unit Atoms: Electron Organization.) Energy circles are full when no more electrons can be in that Energy circle. Atoms are more stable when their outer Energy circle is full.
Some bonding between atoms results from atoms having only one electron in the outer-most Energy circle. To minimize (potential) energy, the electron may move from an Energy circle (or energy level) in one atom to another. Atoms may also share electrons with each other in order to fill up the outer Energy circles.
Bonding Example 2: H2O (water).
Reminder: Atoms "like" having a "full" outer Energy circle.
Bonding Example 3: CO2. In CO2, different atoms "share" electrons. Two of each of the oxygen's electrons (represented as dots below) orbit both the oxygen atom and the carbon atom. Also, four of the carbon atom's electrons (represented as "X"s below) orbit both the oxygen and carbon atoms. So, there is a lot of sharing of electrons in a CO2 molecule! Now, all three atoms have two electrons in the closest Energy circle, and also share 8 electrons in the second Energy circle.
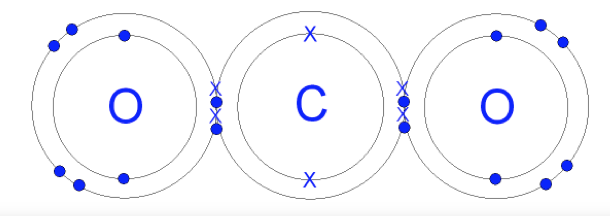
The oxygen atoms only need two more electrons to complete their outer Energy circle. But the carbon atom needs four more electrons to do that. So, the oxygen atoms are closer to having their second Energy circles completed.
Also, oxygen atoms have 8 protons (each with a positive charge). Carbon atoms have 6 protons. So, electrons (which are negatively charged and attracted to positively charged protons) tend to be pulled to of the CO2molecules.
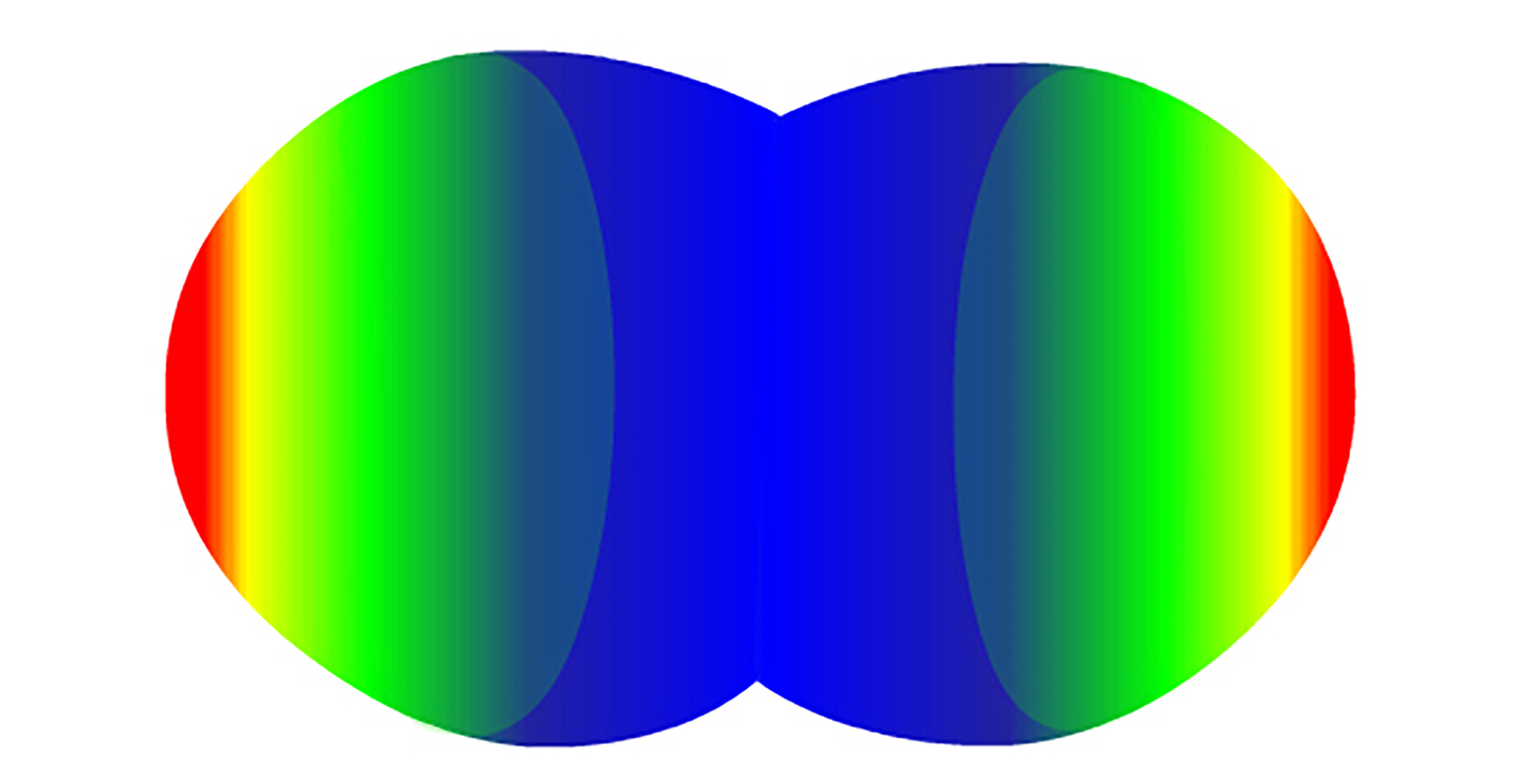
Because the oxygen atoms have more protons and pull harder on the electrons, the shared electrons spend more of their time in the oxygen atoms' Energy circles. As a result, the oxygen ends of the CO2 molecules tend to be more negatively charged (represented as red in Figure 1 below). The carbon in the middle tends to be more positively charged (represented as blue in Figure 1 below).
Figure 1. Charge distribution of Carbon dioxide molecule (red: negative charge; blue: positive charge)
For more information about why atoms "take" or "share" electrons from/with other atoms, please see the unit: How Electrons are organized.
(Slightly Advanced concept: Polarity)
Polarity. When one side of a molecule has a positive charge and the opposite side has a negative charge, the molecule is considered to be "polar." Polar molecules attract the oppositely-charged parts of other polar molecules. Water molecules are polar molecules: the hydrogen sides are positively charged and the opposite (oxygen) sides are negatively charged.
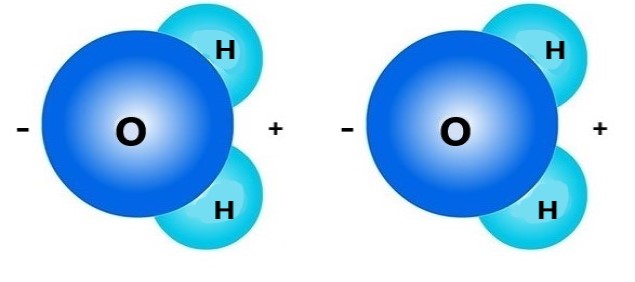
Above are two water molecules. The positively charged side of the first molecule and the negative side of the second molecule attract each other. These attractions allow water molecules to form droplets.
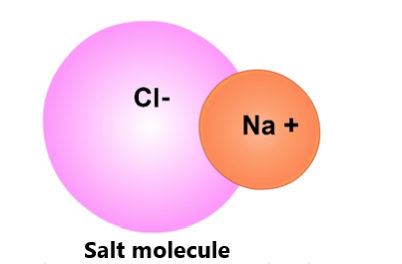
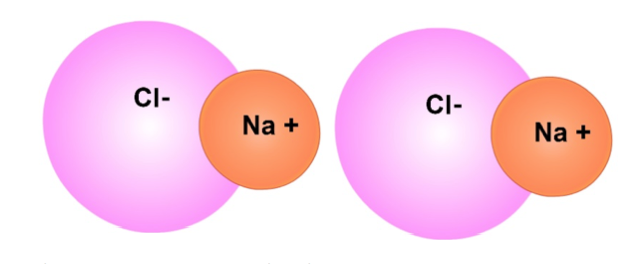
Another example of a polar molecule is table salt (NaCl). The positively charged side of the first molecule and the negative side of the second molecule attract each other. These attractions allow NaCl molecules to form crystals.
You've reached the end of this unit. You can click on the Chemical and Physical Reactions button above for more units related to your research question.