

What is nucleation? Have you ever noticed that when you first fill a pot with water, you often see bubbles on the inside surface of the pot? These bubbles are air that was mixed in with the water when you filled the pot.
Or, if you put your finger in a glass of soda pop or any carbonated drink, you will see bubbles form on your finger. These bubbles are made of the carbon dioxide in the carbonated soda.
If you are a very observant person, you may also have noticed that water first freezes (or becomes ice) on bumpy areas of containers.
All three of these examples involve the process of nucleation. Nucleation is a process where the same type of molecules build up (such as a bunch of CO2 molecules or a bunch of NaCl molecules or a bunch of H2O molecules). Molecules build up on what are called “nucleation sites.” Nucleation sites include places on surfaces that are not smooth, but rather have bumps and/or holes. Your finger is not completely smooth, but has little bumps, including what we call fingerprints. This is why you will see bubbles form on your finger if you put it in a carbonated drink (shown below).
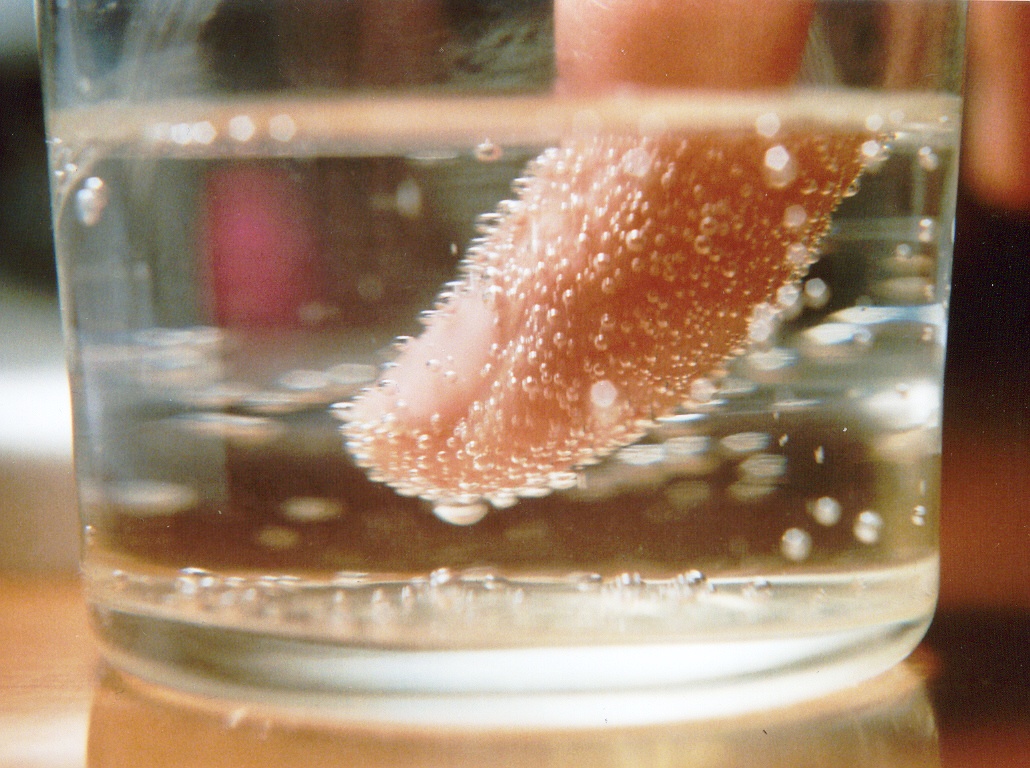
Bubbles will form on your finger when you put it in a carbonated liquid (a liquid with dissolved carbon dioxide). Bubbles of carbon dioxide will build up on the little bumps and ridges on your finger.
Notice that there are few bubbles on the inside of the glass. This is because glass is much smoother than your finger, so there are not as many little bumps and ridges where carbon dioxide bubbles can build up.
Bumps on surfaces are one cause of nucleation. Say you found a pot that had a very smooth surface. You wouldn’t see nearly as many air bubbles form on the inside surface of that pot when you pour water into it. This is because there are fewer nucleation sites on a very smooth surface compared to a rougher surface.
Nucleation sites also help water molecules build up to freeze into ice! In addition to rough spots on surfaces, impurities in water such as dirt particles or salt can also become nucleation sites. Ice begins to form around nucleation sites. In fact, when there are no nucleation sites, water freezes (or becomes ice) at a MUCH lower temperature: approximately -39°C (or -38°F)! (Water normally freezes into ice at about 0°C (or 32°F).) In other words, if water is in a very smooth container, and the water does not have any impurities in it (the liquid is only made of H2O molecules), it will become ice at a much lower temperature.
But most people never come across water that is 100% pure. Even distilled water has a small amount of impurities in it (though much less than tap water).
Exactly why molecules build up on nucleation sites (surfaces with rougher textures and impurities in substances) is not currently well understood. Perhaps someone (maybe you!) will figure this out one day.
Nucleation in Carbonated Liquids (like Soda). Carbonated liquids (such as a soda) contain both water (H2O) and lots of carbon dioxide (CO2) that is dissolved in the water. CO2 gas bubbles form on the nucleation sites, including bumps and other impurities on the surface of the soda container (bottle, can, or glass/cup).
The picture below (on the left) is an image of a kiwi fruit in soda. Kiwis are bumpy and have lots of nucleation sites. So, lots of CO2 molecules will form bubbles on the kiwi surface. The picture below (on the right) is a simplified drawing of CO2 bubbles forming on the bumpy surface of an object in carbonated water. Bumpy objects that can cause nucleation in carbonated water (or sodas) include candies and mints.

The bubbles forming are made of lots of little CO2 molecules. When the bubbles become large enough, they will break away from the nucleation sites. These larger bubbles (shown as blue circles in both pictures above) will rise through the liquid due to the upward buoyancy force.
The CO2 bubbles that have risen from the nucleation sites are made of many many CO2 molecules. Many people think that when you drop a fruit or a mint into a carbonated drink, a chemical process/reaction happens. But what is happening is that the carbon dioxide molecules in the drink are gathering together on the nucleation sites. These CO2 molecules then leave the nucleation sites and move up and out of the drink. The CO2 molecules stay the same. So, these people are mistaken: This is actually a physical process! (Not all cool-looking processes are chemical processes: Many are physical processes.)
Nucleation in carbonated liquids (continued)
When CO2 gas bubbles travel upward through the carbonated liquid, they push up on the liquid that is above. So, if there are more CO2 gas bubbles and/or if the gas bubbles are bigger, more of the liquid above the bubbles will be pushed upward (and, often, out of the can or bottle!).
To leave the liquid and move into the air, the bubbles must be able to break through the surface of the liquid. The water molecules at the surface of the liquid are more strongly attracted to each other than the H2O molecules below the surface. This makes it harder for CO2 bubbles to push through the surface of the liquid (compared to rising to the surface of the liquid).
As discussed in the Evaporation unit, particles (in this case, the CO2 gas bubbles) with more kinetic energy are better able to push through the surface tension of the water and "escape" into the air. This means that both (a) larger CO2 bubbles, which have more mass, and (b) faster-moving bubbles are more likely to push through the surface of the liquid and move into the air above.
The bubbling/fizziness that you may see at the surface of the soda (or other carbonated liquid) is partly due to the surface tension among H2O molecules. At first, the bubbles of CO2 push up on the surface of the soda but do not break the thin layer of H2O molecules. If the CO2 bubbles have enough kinetic energy (mass and/or speed), they will be able to burst through this thin layer of water.
This is much like when you blow bubbles: If you blow too hard (i.e., blow too much air or blow too fast), you will break the thin layer of water and soap and burst the bubble.
For more information about carbonated water and soda, see the unit: Soda (Carbonated water).