

What is Evaporation? Say you are heating a pot of water on the stove (shown below). Thermal energy is produced in the chemical reaction of burning natural gas (which is mostly methane). This reaction produces high-energy (or very fast-moving!) CO2 and H2O molecules. These molecules hit the metal molecules that make up the pot. As a result, the molecules of the pot start vibrating faster. Thermal energy is then transferred between the pot's molecules and from the pot's molecules to nearby water molecules. This causes the water molecules to move around faster and faster. The water's temperature is increasing.

Soon, some water molecules move fast enough to overcome the pull from electric forces of other water molecules around them. They also have to overcome the electric forces of the water molecules at the surface. When they are moving fast enough, the H2O molecules leave the pot and move into the air. These H2O molecules are now called water vapor. This process of going from a liquid to gas state is what we know as evaporation.
When a liquid like water evaporates, the molecules themselves do not change when they move from the pot into the air. They are still H2O molecules (two hydrogen atoms and one oxygen atom). Because the molecules don't change, this is a physical reaction.
Analogies for Evaporation. Evaporation is similar to a rocket blasting off and escaping from the Earth's gravitational field. Rockets need to have enough speed to overcome the pull of gravity and escape from the Earth's atmosphere. To escape into the air (or evaporate), water molecules need to have enough speed to overcome the electric forces of other H2O molecules within the liquid water and especially at the surface of the water (where H2O molecules are even more strongly bonded together).

Another analogy for evaporation is rolling a magnetic steel ball on a table past a magnet. If you roll the ball slowly, the ball will get "caught" in the magnetic field and "stick" to the magnet. But if you roll the ball faster, it will have enough speed to overcome the magnetic attraction and move past the magnet. This is why faster-moving H2O molecules are more likely to evaporate. They can "escape" from the electric forces of the other H2O molecules.
Evaporation of salt water. In the unit on dissolving, we talked about how salt (and also sugar) dissolve in water. But what happens when salt water evaporates? Let's say we have a glass of salt water sitting on a window sill. We forget about it and discover it several weeks later. What will we find?
Most likely, most (if not all) of the water will have evaporated. This happens because eventually, individual water molecules will gain enough kinetic energy (from the sun or surrounding air) to evaporate. But the originally dissolved salt (NaCl) will not evaporate with the water molecules.
Below is a photo of the inside of a glass that had water with salt dissolved in it. This photo was taken after all the water evaporated (about three weeks later). The salt had formed hard crystals along the inside of the glass as the water evaporated.

Why does the salt stay behind? One reason that the NaCl molecules will not evaporate even though H2O molecules do is because they are much heavier than H2O molecules. If we look at the Periodic Table of Elements, we see that the atomic mass of Na is 23 "atomic mass units" or amu. And Cl is about 35 amu.
In H2O, there are two H atoms. Each H atom is only 1 amu. There is one O atom, which is 16 amu.
Because NaCl molecules are heavier, they are pulled downward by the Earth's gravity more strongly than H2O molecules. So, they do not escape from the glass and evaporate into the air.
What is Melting? When thermal energy is transferred to ice, the H2O molecules begin to vibrate faster and faster. Some of the molecules have enough energy to overcome their bonds with nearby H2O molecules. These molecules are no longer fixed in place in the ice crystal (see Crystals unit) and can freely move about. These molecules are now in liquid form. If enough thermal energy is transferred to the ice, the ice will melt completely and become liquid water. In its liquid state, the H2O molecules are now moving around independently, kind of "slithering" past each other. But there are still attractions between H2O molecules because of electric forces (see the Atoms Unit for more information).
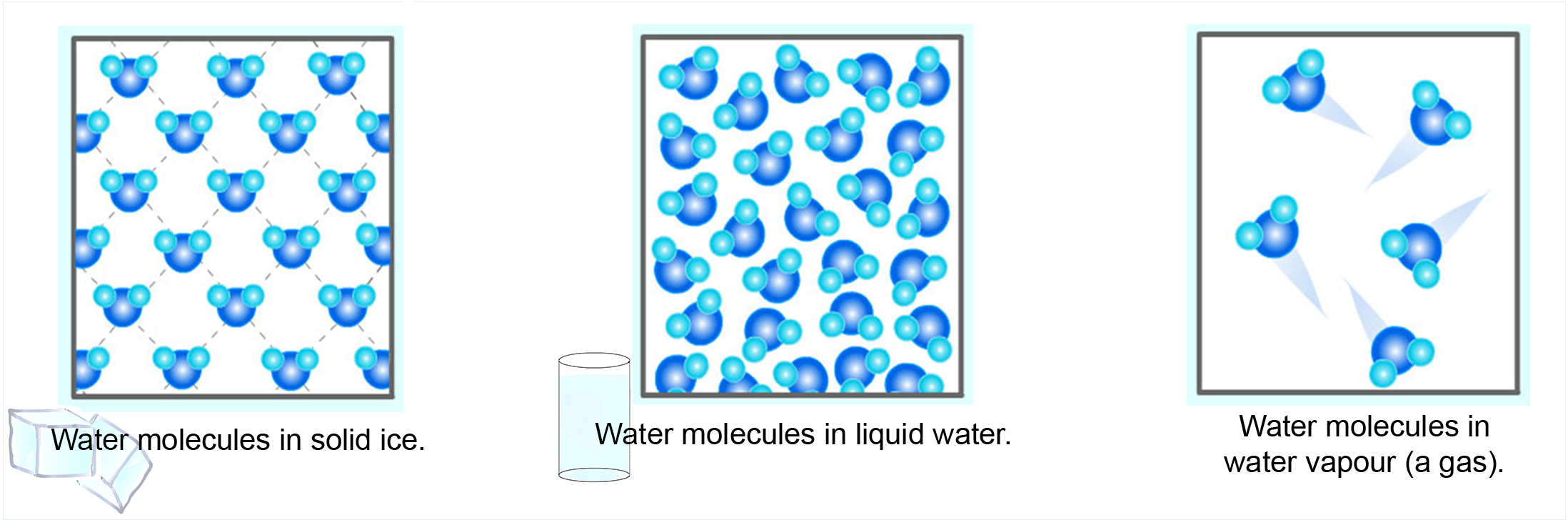
This simulation below shows the motion of molecules and the distance between the molecules when they go from a solid to liquid to gas state. The molecules are represented as bright blue circles below. They are magnified by a lot so we can see them in this simulation.
Phet States of Matter Simulation (click above to view)

Here are some important things to understand (that many people don't):
Check your final understanding...