

Forms of energy. There are many different forms of energy from different sources: e.g., radiation from the sun, (kinetic) energy from the wind, thermal energy from heat sources, and (kinetic) energy from moving water. Remember that energy is related to motion.



Energy can convert (or change) from one form to another. For example:
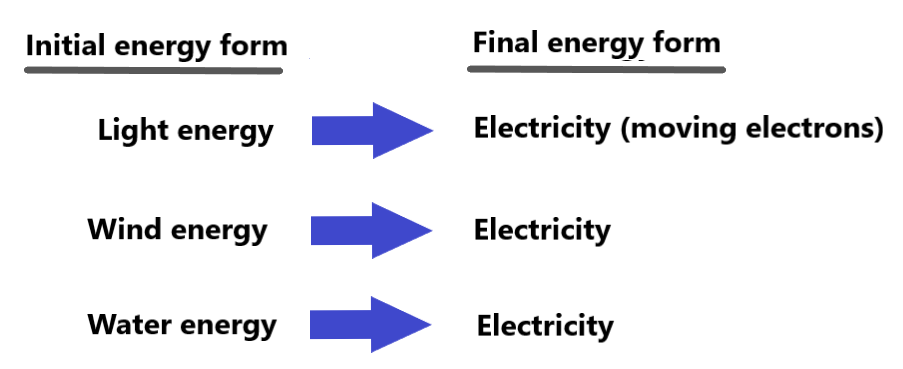
This allows us to use the energy from sources like light, wind, and kinetic energy of water to generate electricity (movement of electrons along a wire or other material) that we use for lots of things! Some devices that use electricity are shown below.

These devices in turn convert electricity to other forms of energy, including light, sound, and air movement.
When energy is converted to a different form, the total amount of energy must remain the same. This is a very important scientific principle called Conservation of Energy. According to the principle of conservation of energy, the total amount of energy always stays the same, including when energy is converted to a different form!
In the following pages, we'll first talk in more detail about how energy is converted from one form to another. After that, we'll talk about how energy is conserved when it changes forms...
Examples of energy changing forms:

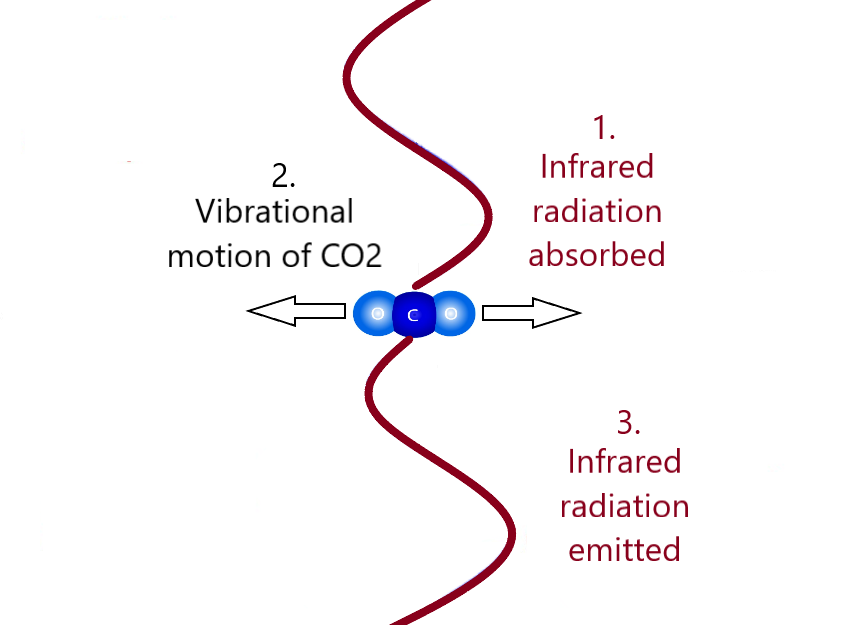
Energy is conserved! In all of these examples, the total amount of energy before the conversion is equal to the total amount of energy afterward.
Check your understanding...
A solar oven (like the one shown below) converts energy from the sun's radiation into thermal energy. Radiation from the sun passes through the transparent plastic wrap covering the oven. The radiation causes molecules of the air, food, and anything else in the "oven" to move faster. This results in the temperature of the oven increasing.

Application question: Can you see any ways to improve this oven to make its temperature even higher?
Energy is conserved! (This is important!) Anytime that energy changes forms, the total amount of energy before the conversion is equal to the total amount of energy afterward.
Conversion of Kinetic and Potential Energy. Another way energy changes forms is between kinetic energy and potential energy. But the total amount of energy must remain the same at all times: this is the principle of Conservation of Energy
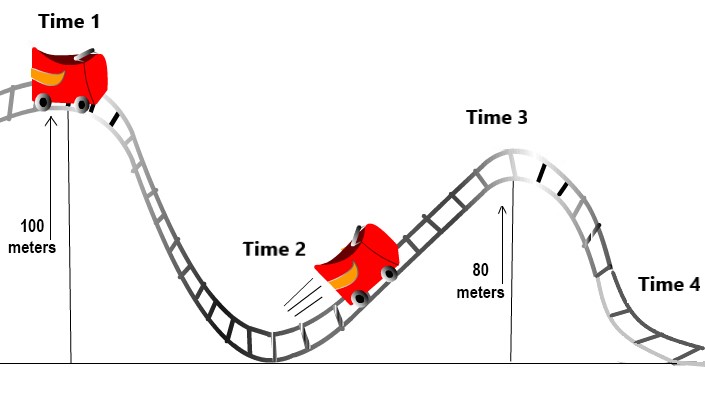
 Fill in the blanks!
Fill in the blanks!
The potential energy of the roller coaster at Time 1 is its potential energy at Time 2.
The potential energy of the roller coaster at Time 2 is its potential energy at Time 3.
And, the potential energy of the roller coaster at Time 3 is its potential energy at Time 4.
The kinetic energy of the roller coaster at Time 1 is its kinetic energy at Time 2.
The kinetic energy of the roller coaster at Time 2 is its kinetic energy at Time 3.
And, the kinetic energy of the roller coaster at Time 3 is its potential energy at Time 4.
The total energy of the roller coaster (kinetic + potential energy) at Time 1 is its kinetic energy at Time 2 (it has no potential energy at Time 2).
The total kinetic energy of the roller coaster (it has no potential energy) at Time 2 is the sum of its kinetic and potential energy at Time 3.
And, the total energy of the roller coaster (kinetic + potential energy) at Time 3 is its kinetic energy at Time 4 (it's at the bottom of the hill and has no potential energy).
The Conservation of Energy principle is shown in the animation below. The total amount of energy, which is represented by the sum of the heights of the red bars, is always the same. Energy just converts from kinetic to potential energy and from potential to kinetic energy.

Check your understanding...
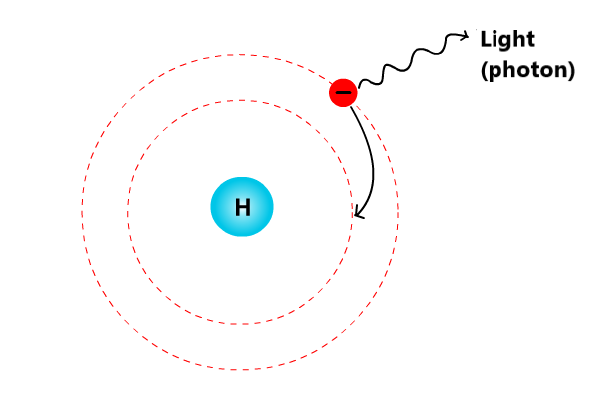
We just saw how the energy of electrons can be converted into light energy. But the reverse process can also happen: Light energy can be converted into kinetic energy of electrons. Light may hit electrons in certain elements (such as the metals cesium and potassium) and molecules (such as chlorophyll molecules), causing the electrons to escape from the atoms or molecules. As represented below, the amount of kinetic energy of the escaped electrons is related to the energy of the light the electrons absorb. Electrons that absorb higher-energy blue or violet light will have more kinetic energy than electrons that absorb lower-energy green light. Red light, which has even less energy than green light, doesn't have enough energy to allow electrons to escape from the metal.
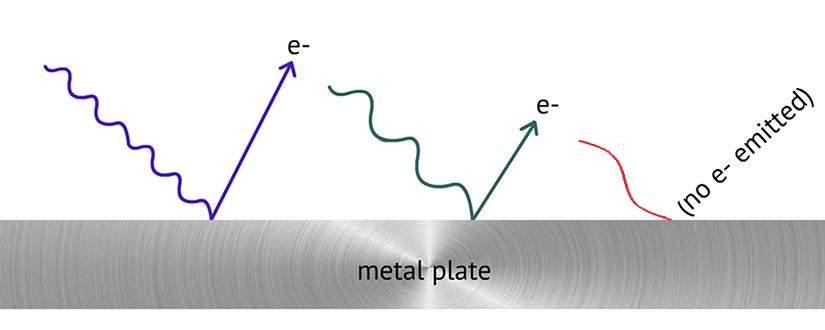
In both cases (when electron's energy is converted into light energy OR light energy is converted into electron energy), the total amount of energy is the same before and after the conversion.