

What happens to something when it dissolves? When you add sugar to a drink like lemonade or iced tea and mix it around, the sugar will dissolve in the drink. This means that the little grains of sugar will break into smaller and smaller sugar particles. Eventually, the sugar may break down into single molecules of sugar and spread throughout the drink.
For example, as shown below, brown sugar was added to a glass of water. At first it sank to the bottom of the water (as shown below, left). After the water was stirred, the brown sugar is no longer visible. It has dissolved, or broken down into particles that are too small to see. In the picture below and to the right, you can no longer see the brown sugar after it has dissolved in the water.


In general, a solid substance added to a liquid dissolves when the atoms or molecules of the solid break away from the solid substance and become spread throughout the liquid.
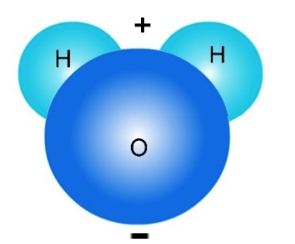
(See molecules unit for a review of H2O and NaCl molecules.)
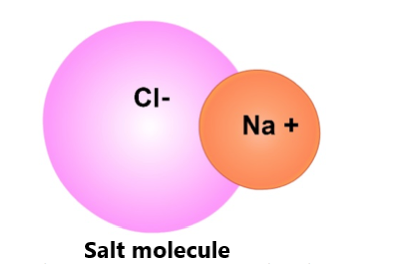
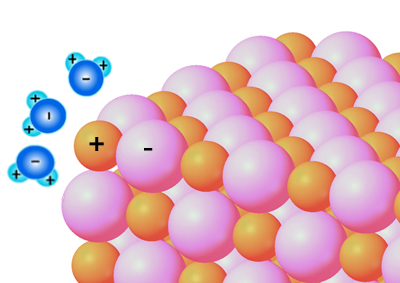
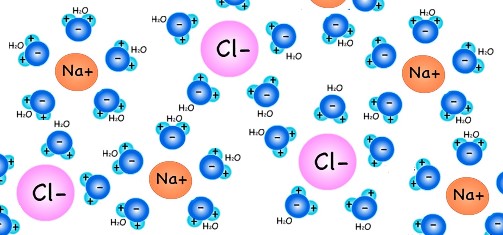
Salt dissolving in water. When table salt (NaCl) is added to water, some H2O molecules will pull on the atoms of salt (both the positively charged sodium, Na+ ions and negatively charged chlorine, Cl- ions) that are on the outer surface of the salt crystals. The negatively charged sides of the water molecules will surround the Na+ ions (which is positively charged (+) because it had lost its electron to the Cl atom) and pull the Na+ ions away from the Cl- ion.
Similarly, the positive sides of H2O molecules surround the negatively charged Cl- ions and pull the Cl- ions from the Na+ ions. This is how the Na+ and Cl- ions break away from the NaCl crystals and spread throughout the water.
NaCl Dissolved in water (H2O)
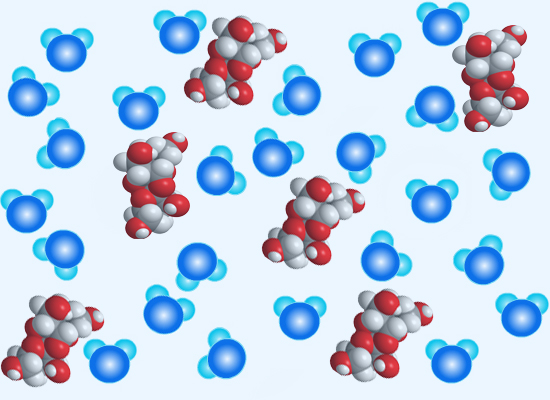
Sugar dissolving in water. When sugar is dissolved in water, some H2O (water) molecules will bond with the molecules of sugar that are on the outer surface of the sugar crystals. C12H22O11 is the chemical formula for the molecules that make up both brown and white sugar. The oppositely-charged areas of the water and sugar molecules will attract each other. If the electric forces that cause the H2O-sugar molecule bondings are "strong" enough, the sugar molecule will be "freed" from its bonds with other sugar molecules and move about in the water. This is how sugar crystals dissolve in water.
The picture below represents sugar molecules (white/red) dissolving in water (water molecules are darker/lighter blue).
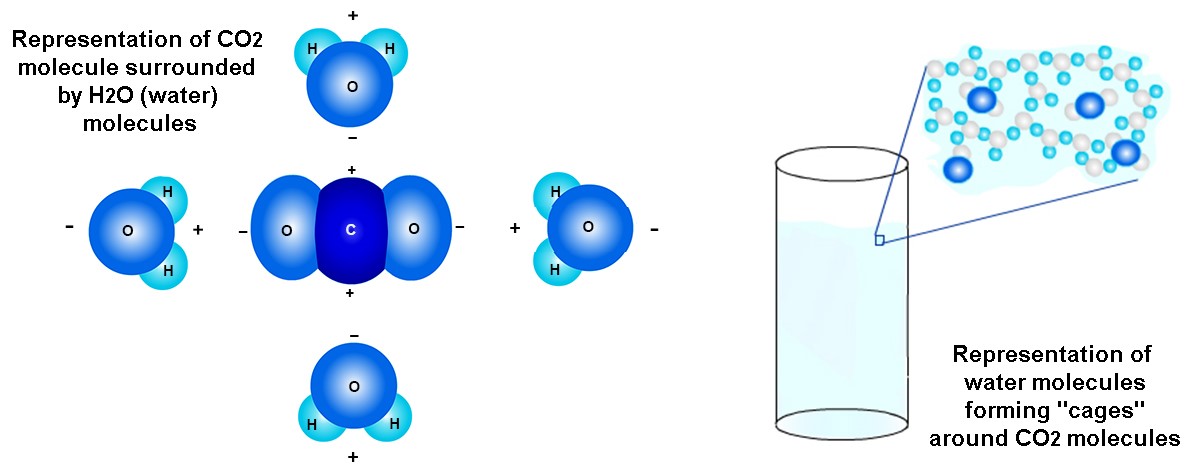
Carbon dioxide dissolved in water. Carbon dioxide (CO2) molecules can be dissolved in liquids, including in water. This causes the "fizziness" of drinks like soda. CO2 can dissolve in water because CO2 and H2O molecules are attracted to oppositely charged parts of each other. These attractions help to "hold" the CO2 in the water. The picture below on the left shows a simplified image of how electric forces from H2O molecules may "hold" CO2 in the liquid. Note that oppositely-charged areas of the CO2 and H2O molecules are attracted to each other. The picture below on the right shows a slightly more complex image of how H2O molecules might surround CO2 molecules in the water. Some people say that the H2O molecules form "cages" around each CO2 molecule.
CO2 is also kept in the soda because there is a high pressure from the air above the soda in the bottle before it is opened. This high pressure helps keep the CO2 that is near the surface of the soda from "escaping" the liquid and into the trapped air in the bottle.
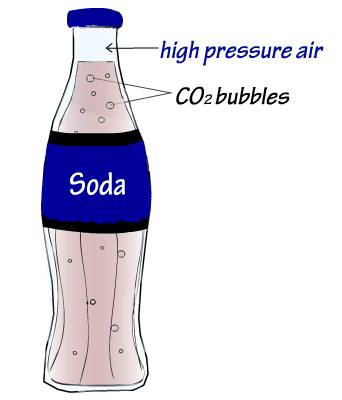
Once you open the bottle, the high-pressure air at the top of the bottle is released into the (lower-pressure) atmosphere. As a result, there is now less pressure from air at the surface of the soda. Because of this, some CO2 may be able to "escape" from the soda. When this happens, sometimes enough CO2 escapes that the CO2 also pushes some of the bubbly liquid out with it. This has probably happened to you (from time to time) when you open a bottle of soda.
When you shake a bottle of soda before opening it (which I do not recommend!), some of the CO2 molecules will group together to form larger bubbles in the soda. When this happens, the CO2 bubbles have more mass and are better able to break through H2O--H2O bonds in the liquid above them to escape into the atmosphere. This is why when you shake the soda bottle before opening it, the soda will probably "explode" out of the bottle. You'll have a big mess to clean up!
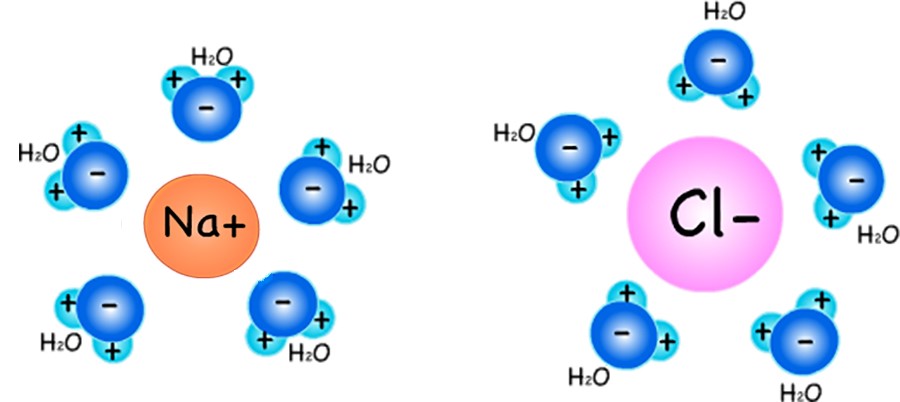
Saturation point. There is a limit to how much of a substance (such as table salt, NaCl) can dissolve in water. For example, if you are adding salt to water in a glass, and keep stirring the water, eventually no more salt will break up and dissolve. This is called the saturation point. The saturation point in salt water happens when there are JUST enough H2O molecules to surround and hold up all the (heavier) Na+ and Cl- atoms in the water. (This idea is represented below.)
When the concentration of salt in water is greater than the saturation point, the extra Na+ and Cl- atoms that are NOT surrounded by water molecules rejoin to form NaCl molecules. If the concentration of salt in water is much higher than the saturation point, you will start to see salt crystals building up. This buildup may be at the bottom of the glass (since NaCl is more dense than H2O molecules) or places that have little bumps (nucleation sites).
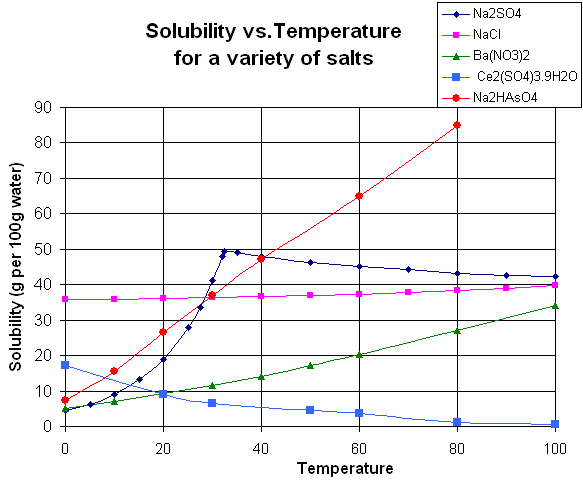
The graph below shows the relationship between water temperature and the "solubility" of different types of salts. The solubility of the salt is how much of the salt (in grams) can be dissolved in 100 grams of water (at that water temperature).
Let's think more about what happens to a glass of salt water after 3 weeks...